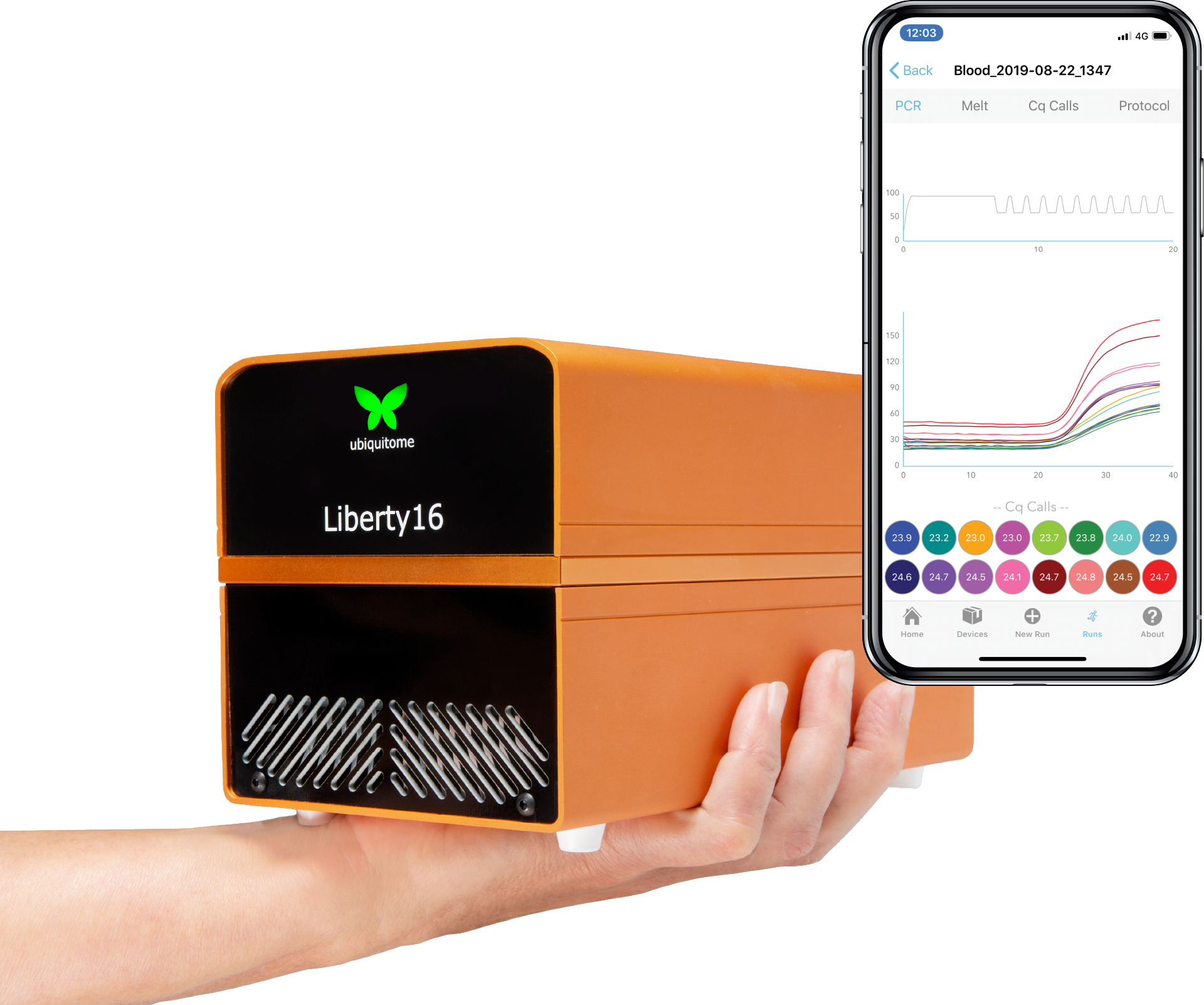
Ubiquitome connects the world to meaningful genetic information through portable, cloud-connected, proven qPCR devices
Read more: FDA-EUA SalivaDirect Liberty16
Small | Portable | Low Cost | Accessible | Proven
Ubiquitome - Portable Thermal Cycler PCR Machine
Imagine a world where powerful PCR testing is readily available at your fingertips. Ubiquitome makes this a reality. We connect the world to meaningful genetic information through portable, cloud-connected, and proven portable molecule testing machines.
Get fast, accurate results in the field, on the farm, or even in your clinic. Learn more about Ubiquitome and the Liberty16, our revolutionary portable PCR device, below.
Who Is Ubiquitome?
Achieving high-quality results while minimising costs
Conventional lab-bound methods often lead to delays in disease diagnosis. At Ubiquitome, our mission is to transform the fields of life science, biotech, and healthcare by providing cutting-edge solutions to tackle crucial challenges. Our revolutionary portable PCR machine offers a portable, efficient, and gold-standard solution for molecular testing, particularly in resource-limited settings. Our device has received FDA-EUA approval for the SalivaDirect protocol, enabling the detection of SARS-CoV-2 RNA, the virus responsible for COVID-19.
This is just the beginning. We're committed to continuous improvement, making portable PCR a reality for everyone. With substantial investments in optimising our manufacturing and supply chain processes, we're excited to reveal that our portable PCR machine price is now significantly lower. This marks a significant milestone in our efforts to make our technology more accessible and impactful in healthcare.
Revolutionise your approach to disease detection; explore how our portable qPCR machine can transform your research, veterinary practice, or business.


INTRODUCING THE LIBERTY16
Stay ahead of the curve with the mobile, real-time PCR machine thermocycler that fits in the palm of your hand. The Liberty16 app drives the mobile Liberty16 handheld real-time PCR device for answers in as little as 20 minutes, with various other features:
- Liberty16 connectivity provided via Bluetooth©.
- Simple 30 second real time PCR protocol set-up and run.
- Dynamic monitoring of annotated real time PCR amplification curves.
- Automated Cq calling, eliminating the need for human intervention.
- Share raw run data and Cq calls via email and upload them to iCloud.
Download the iPhone app to see how it works.
Small
A truly hand-held qPCR device, perfect for being on the go
Mobile
Operates for up to three hours on a re-chargeable battery
Cloud - enabled
Connect to iPhone app via Bluetooth to access test results
Fast
Gold-standard results in less than 20 minutes
Take Control of Disease Detection
Portability Offers Unmatched Convenience
Ubiquitome designs our handheld real-time PCR devices to accommodate your needs and will fit into whatever space you have to work with, wherever you are. Our easy-to-use, open platform has you covered for your different PCR testing solutions.
Compact & Convenient
Our handheld, battery-powered devices provide unparalleled portability compared to bulky traditional lab equipment
Unmatched Flexibility
Test a wide range of samples, including human, animal, and plant samples, for various diseases using qPCR assays.
Fast and Efficient
Get results within 30 min to 1-2 hours, depending on the assay, allowing for swift decision-making.
Durability Built-In
Designed for field use, our small PCR machines can withstand the demands of working outside traditional lab settings.
Custom Solutions
Ubiquitome can develop custom assays for clients wanting to test for specific pathogens.
Portable Thermal Cycler Applications
Clinical
Our portable PCR machine enables precise and rapid detection of nucleic acids to identify pathogens and genetic variations.
Environment
Liberty16 aids in the rapid and accurate assessment of microbial contaminants or pollutants in various environmental samples.
Agriculture
Identify genetic traits and monitor plant health with our mobile PCR machine, enabling precise and efficient management of crops and livestock.
Publications
Explore the following publications to discover how our PCR device is revolutionizing the way we understand, diagnose, and combat diseases.
Abstract
The COVID-19 pandemic has highlighted the need and benefits for all communities to be permitted timely access to on-demand screening for infectious respiratory diseases. This can be achieved with simplified testing approaches and affordable access to core resources. While RT-qPCR-based tests remain the gold standard for SARS-CoV-2 detection due to their high sensitivity, implementation of testing requires high upfront costs to obtain the necessary instrumentation. This is particularly restrictive in low-resource settings. The Ubiquitome Liberty16 system was developed as an inexpensive, portable, battery-operated single-channel RT-qPCR device with an associated iPhone app to simplify assay set-up and data reporting. When coupled with the SalivaDirect protocol for testing saliva samples for SARS-CoV-2, the Liberty16 device yielded a limit of detection (LOD) of 12 SARS-CoV-2 RNA copies/µL, comparable to the upper end of the LOD range for the standard SalivaDirect protocol when performed on larger RT-qPCR instruments. While further optimization may deliver even greater sensitivity and assay speed, findings from this study indicate that small portable devices such as the Liberty16 can deliver reliable results and provide the opportunity to further increase access to gold standard SARS-CoV-2 testing.
Keywords: COVID-19; PCR; SARS-CoV-2; diagnostics; saliva.
Abstract
A significant limitation of quantitative real-time polymerase chain reaction (qPCR) technology for environmental research has been the lack of portability of the amplification and detection equipment needed to perform qPCR assays in the field. The ability to perform qPCR assays in the field would significantly improve the utility of the technology, enabling quicker risk management decisions in acute environmental emergencies, and speeding the pace of environmental research. We tested a portable qPCR instrument, Liberty16, to evaluate if this instrument would be useful for qPCR analysis in our fieldwork. Parallel testing of the Liberty16 with our existing LightCycler 480 Instrument II revealed similar repeatability and sensitivity for the detection of targets in known and blind freshwater samples by qPCR. Both instruments were able to detect all target sequences at 20 copies per reaction and up to 66% targets in a 10-fold dilution. In seven of eight blind freshwater samples, results from both instruments were consistent for detected or nondetected target. In the other sample, the target was only detected by the Liberty16. Portable instruments of this type may open up new avenues for researchers to perform qPCR analyses in the field.
Abstract
African swine fever (ASF) is a contagious viral hemorrhagic disease that affects domestic pigs and wild boar. The disease is notifiable to the World Organization of Animal Health (WOAH), and causes significant deaths and economic losses. There is currently no fully licensed vaccine available. As a result, early identification of the causative agent, ASF virus (ASFV), is crucial for the implementation of control measures. PCR and real-time PCR are the WOAH-recommended standard methods for the direct detection of ASFV. However, under special field conditions or in simple or remote field laboratories, there may be no sophisticated equipment or even stable electricity available. Under these circumstances, point-of-care systems can be put in place. Along these lines, a previously published, rapid, reliable, and electricity-free extraction method (TripleE) was used to isolate viral nucleic acid from diagnostic specimens. With this tool, nucleic acid extraction from up to eight diagnostic samples can be realized in one run in less than 10 min. In addition, the possibility of completely omitting viral DNA extraction was analyzed with so-called direct real-time PCR protocols using ASFV original samples diluted to 1:40 in RNase-free water. Furthermore, three real-time PCR cyclers, developed for use under field conditions (IndiField, Liberty16 and UF-300 GenecheckerTM), were comparatively applied for the sensitive high-speed detection of ASFV genomes, with overall PCR run times between 20 and 54 min. Depending on the viral DNA extraction/releasing method used and the point-of-care cycler applied, a total time for detection of 30 to 60 min for up to eight samples was feasible. As expected, the limitations in analytical sensitivity were positively correlated to the analysis time. These limitations are acceptable for ASFV diagnostics due to the expected high ASFV genome loads in diseased animals or carcasses.
Keywords: African swine fever virus; DNA isolation; point-of-care (POC); portable real-time PCR.
Abstract
To assist the global eradication of peste des petits ruminants virus (PPRV), a molecular test for the rapid and reliable detection of PPRV was developed which additionally enables the detection of pathogens relevant for differential diagnostics. For this purpose, the necessary time frame of a magnetic bead-based nucleic acid extraction protocol was markedly shortened to 7 min and 13 s. The optimized extraction was run on a BioSprint 15 platform. Furthermore, a high-speed multi-well RT-qPCR for the genome detection of PPRV and additional important pathogens such as Foot-and-mouth disease virus, Parapoxvirus ovis, Goatpox virus, and Mycoplasmacapricolumsubsp.capripneumoniae was established and combined with suitable internal control assays. The here-described qPCR is based on a lyophilized master mix and takes only around 30 to 40 min. Several qPCR cyclers were evaluated regarding their suitability for fast-cycling approaches and for their diagnostic performance in a high-speed RT-qPCR. The final evaluation was conducted on the BioRad CFX96 and also on a portable Liberty16 qPCR cycler. The new molecular test designated as "FastCheckFLI PPR-like", which is based on rapid nucleic acid extraction and high-speed RT-qPCR, delivered reliable results in less than one hour, allowing its use also in a pen-side scenario.
Keywords: Small ruminant morbilli virus; differential diagnosis; fast extraction; high-speed RT-qPCR; molecular pen-side test; peste des petits ruminants virus (PPRV); rapid detection method.
Testimonials
-
Completed first full run on 100% battery power. The last 30mins was in my Jeep on the highway and all worked perfectly.
EDP Biotech
-
Costly instruments for real-time PCR analysis often stand in the way of equitable access for under resourced communities and countries and sustainability of testing systems
Yale School of Public Health Dr Anne Wyllie
-
A simple design of minimal buttons, combined with a sleek aesthetic, make it (Liberty16) both intuitive and fun to operate
Renegade Bio
-
Pretty quick, not very complicated, easy to implement into lab, user-friendly
Altius Diagnostics
-
Our trainers gave us confidence, as non-medical people, that this device could do what we needed it to and was a viable solution in our private workplace
Napier Port Adam Harvey
-
Immense gratitude and praise to the technology provided, allowing Ballance to provide a safer working environment for employees and staff, and above all else – business continuity
Ballance Sean Donovan

Freedom to Choose Grant Program Winners
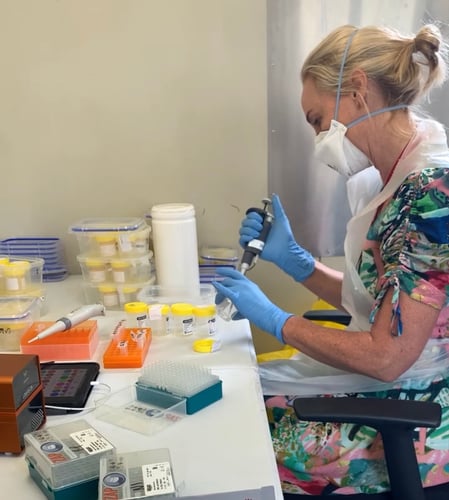
Liberty16 Workplace Surveillance Testing
Now available for small and medium sized workplaces, with affordable on-site gold standard PCR testing to keep costs down, productivity up.
Smaller
Battery-powered
Cloud-enabled
Speed to result
"Costly instruments for real-time PCR analysis often stand in the way of equitable access for under resourced communities and countries and sustainability of testing systems"
Dr Anne Wyllie, Yale School of Public Health (on why they partnered with Ubiquitome)
Our Partners










Contact Us
Please use the form to contact us with any questions, technical inquiries, or to request a demonstration. A Ubiquitome representative will contact you promptly.