Liberty16 Workplace Surveillance Testing for COVID-19
Now available for small and medium sized workplaces
Affordable on-site gold standard PCR testing to keep costs down, productivity up and your staff COVID safe.
Peace of Mind
For you and your employees with regular on-site workplace PCR surveillance testing
Convenient
Provide a saliva sample on arrival to your workplace and receive a result before mid-day
Affordable
Save with testing materials costs as low as US$1/staff member surveillance test
3 Step Liberty16 PCR Surveillance Testing Process
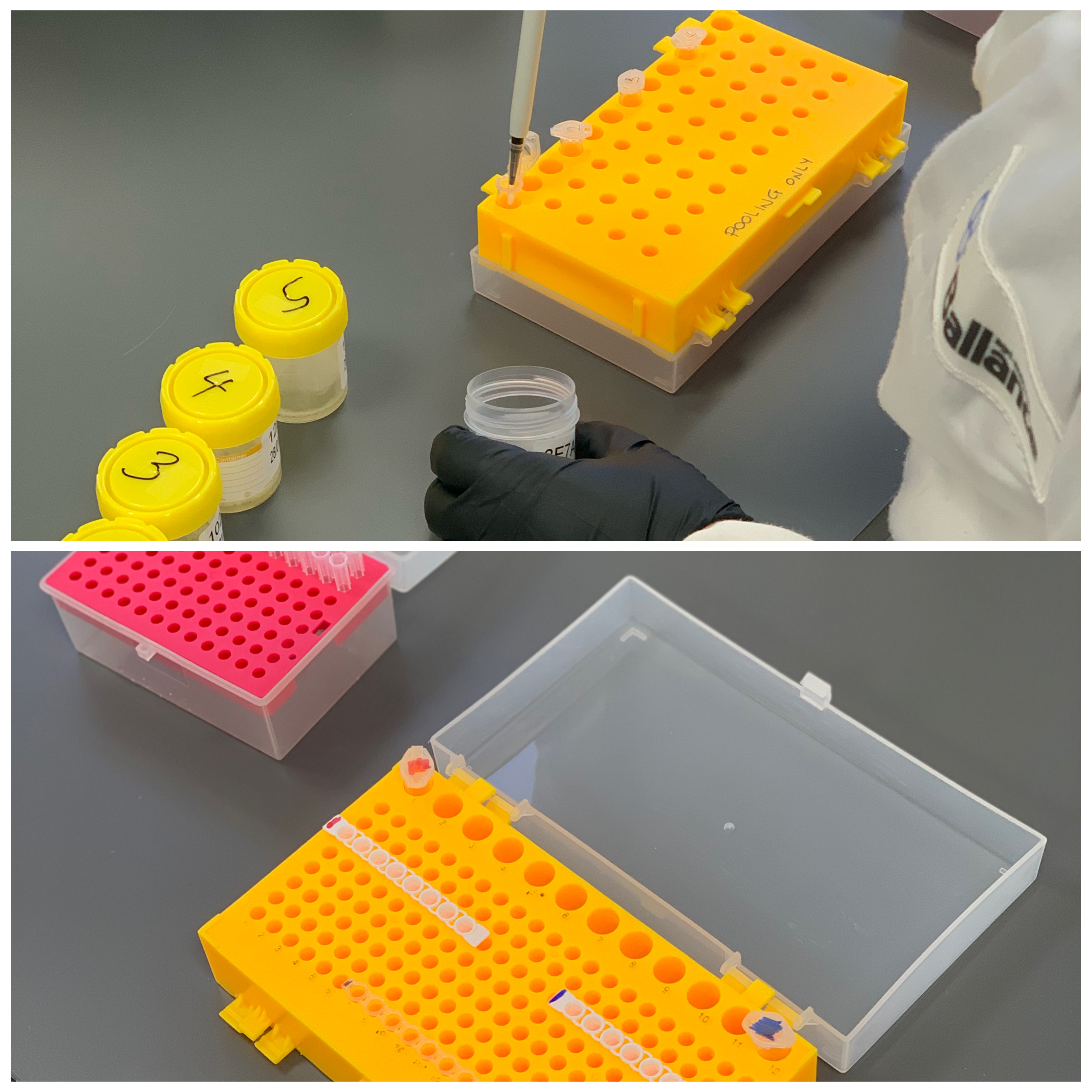
Step 1 - Saliva Collection & Solution Preparation
Participants for the COVID-19 Surveillance Test will be asked to provide their own saliva sample into a provided collection container.
1. Saliva samples from up to 5 different individuals will be combined together creating a 'pool'.
2. Solutions are created from a combination of three ingredients and stored at 4°C until use.
3. Using an 8-strip PCR strip tube and cap, pools are mixed together with the solutions made earlier and analysed within the Liberty16 Surveillance Testing system.
4. This step takes approximately 20 – 30 minutes, depending on operator experience.*
*In the event of process uncertainty, the team at Ubiquitome are committed to assisting when and where necessary, using methods of communication tailored for the customers' needs.

Step 2 - Saliva Processing
Recipes/PCR protocols are programmed within the Liberty16 Apple App - compatible on iPhone/iPad with iOS Version >13.0.
Initiate the protocol with the PCR strip tubes inserted and results will be observable in ~80 minutes.
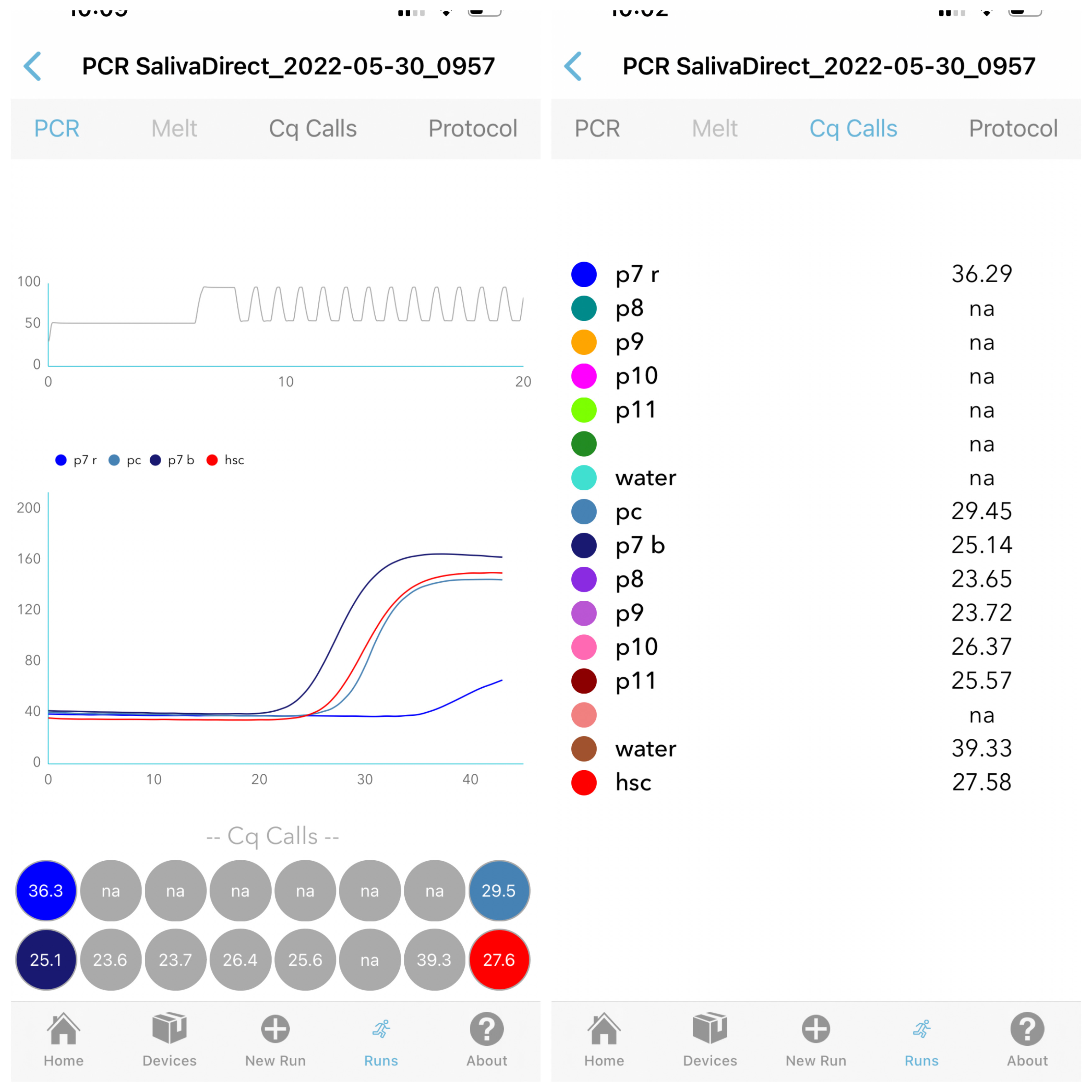
Step 3 - Results
PCR curves reliably and accurately generated using Ubiquitome's built-in algorithm.
A tabled view of Cq (Ct) values for simple interpretation of test results, saliva quality and contamination.
Saliva PCR vs RAT - what's better?
Fast does not always mean better, especially when it comes to Rapid Antigen Tests (RAT). RATs are known for high false negative rates1, 2 , meaning infectious individuals are missed and potentially continue spreading COVID-19 within the workplace.
The Liberty-16 and SalivaDirect™ saliva-based PCR test was designed to be an affordable, non-invasive and long term solution.
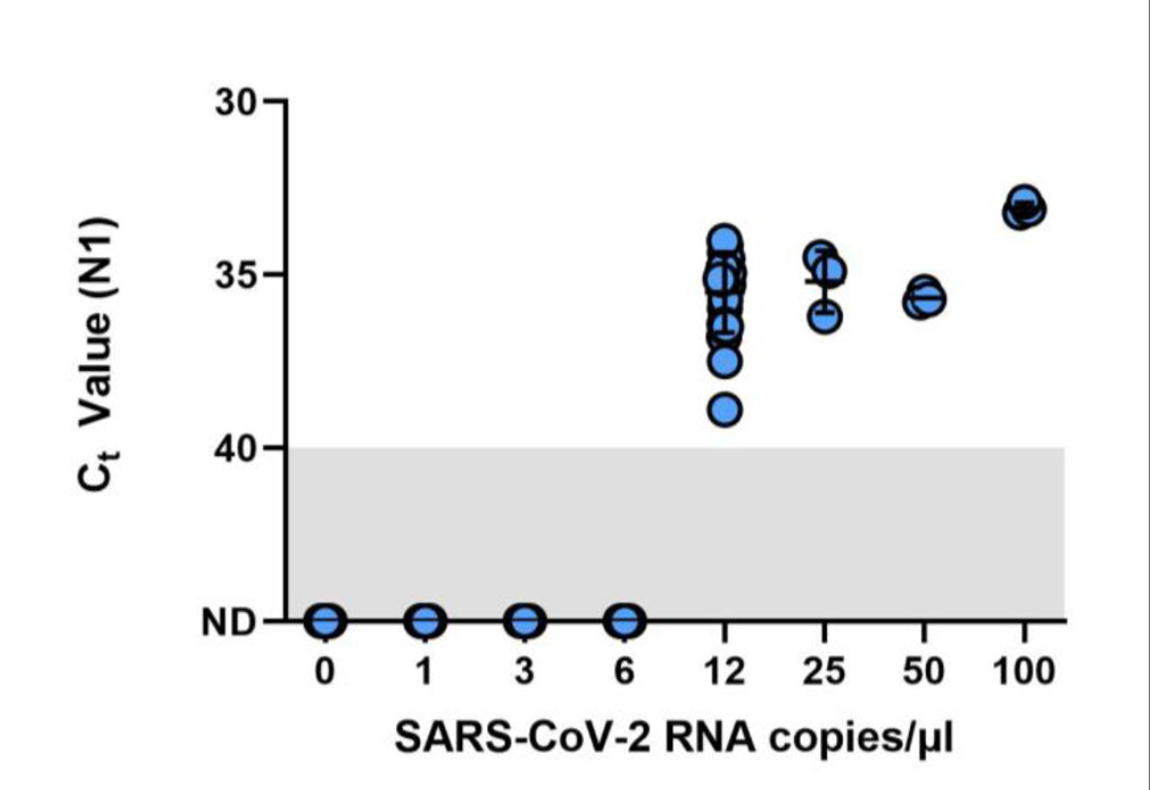
This figure shows results of the Limit of Detection (LOD) finding study which used spiked saliva samples with known concentrations of SARS-CoV-2. The LOD for Omicron on the Liberty16 system was confirmed as 12 copies/μL3.
“We can not thank the people over at Ubiquitome enough for all the support, remote and on-site, and in doing so protecting our community, workers and apples”
Kelvin Taylor, Founder, Taylor Corporation
Guiding you on how to set up your own on-site surveillance testing system
To order a Liberty16 mobile real-time PCR system:
Add it to your cart, enter your details and we'll be in touch with the next steps.
Important purchaser notice
For research use only. Liberty16 may only be used for SARS-CoV-2 diagnostic purposes under an applicable FDA EUA.
This surveillance solution is only available to customers based in the USA who wish to perform COVID-19 surveillance testing at the population level without reporting individual results.
The on-site setup will require more equipment than just the Liberty16 unit. At Ubiquitome, we currently supply the Liberty16 real-time PCR units and we have the resources available to help connect you with the required local suppliers of all necessary supplies.
Register interest
Liberty16 and saliva testing - Frequently asked questions (FAQ)
You will need to assign an operator to perform the surveillance testing.
While employing someone with previous lab experience will be advantageous, it is not entirely necessary as the processes involved can be taught to anyone with an aptitude for learning, an eye for detail and a careful nature.
A competent operator can typically perform up to 4 runs on a single device per day (~120 individuals tested, if pooling), this is scalable by adding more devices and will enable multiple parallel runs.
It depends on your site and the people you are surveilling. One operator can typically perform up to 4 runs on a single device per day, this is scalable by adding more devices that will enable multiple parallel runs, for example: with a second Liberty16 device the technician can start the next run of samples while the first device is still analysing.
Once ordered we can have your Liberty16 unit(s) to you within 1-2 weeks, provided we have stock.
We can have your team set up for surveillance testing in as little as three weeks.
The expected materials cost is approx. ~US$1 per individual tested (if pooled testing of 5 individuals is carried out).
We'll provide you with a trusted supply chain list for ongoing material.
The Liberty16 units are designed and manufactured in New Zealand.
Given the global requirement for COVID testing consumables/testing solutions, please allow a 2-3 week lead time in your supply chain management.
Surveillance testing should take place in a remote or isolated room that is well-ventilated for the best outcome. Prior to purchasing your Liberty16 unit we strongly suggest undergoing thorough space analysis. Your space should account for social distancing, isolated collection, and processing areas.
All of the above can be discussed and reviewed in depth with one of our Application Scientists, at your request.
If pooling: 30 individuals tested per run
No, CLIA certification is not required for this testing as long as it is not used for diagnostic purposes and no individual result will be provided to either the individual or their healthcare provider. Surveillance testing is performed on de-identified specimens as outlined by the CDC4. The FDA does not generally regulate surveillance testing5.
Follow CDC and local public health advice.
You may also like
Ubiquitome gets FDA authorization with Yale SARS-CoV-2 for Liberty16 Pro
19 December, 2022NIH RADx winner’s next gen Liberty16 Pro gets EUA Auckland, December 19, 2022: Ubiquitome’s next generation real time PC...